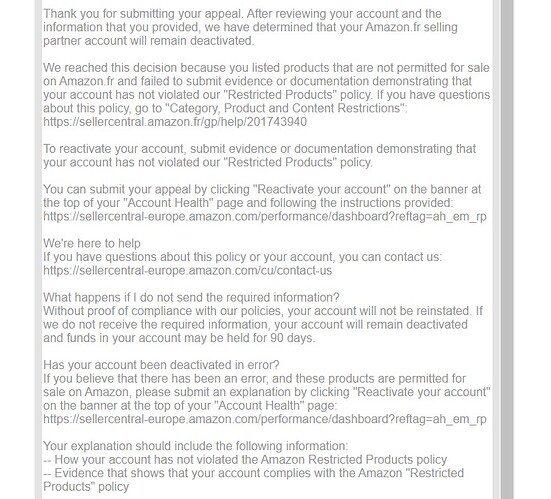
Thank you very much for your Help @Kika
Today we submit new PoA and we get same answer:
Dear Amazon Seller Performance Team,
I appreciate the opportunity to appeal the suspension of my seller account. I take full responsibility for this violation and have developed a Plan of Action to rectify the issue and prevent any future violations.
A) What went wrong:
We failed to review Amazon’s guidelines and the relevant laws concerning the resale of drugs and dietary supplements, particularly the Restricted Product policy.
The lack of verification of the active ingredients included in the composition of the product led to the listing of the prohibited product containing CBD (Cannabidiol) in our inventory and a violation of Amazon’s policy.
• ASIN: B003QYYMUG
• Title: Pommade naturelle soins de douleurs articulaires.
B) Actions taken to fix the problems:
Immediately upon notification, all listings were closed, removal orders were initiated, and all relevant products were removed from my inventory.
• ASIN: B003QYYMUG, Title: Pommade naturelle soins de douleurs articulaires.
• Removal Orders ID: 240801KZW_0
I have diligently studied and fully comprehended Amazon’s policies, guidelines, and help pages, with particular emphasis on those pertaining to Drugs, Drug Paraphernalia and Dietary Supplements and Category, Product and Content Restrictions.
https://sellercentral.amazon.de/help/hub/reference/G201743940
https://sellercentral.amazon.de/help/hub/reference/G201743990
A thorough inventory check was conducted across all Amazon Marketplaces and our personal warehouse to ensure compliance.
All products for which we do not have information on the active ingredients especially about the containing of CBD, or cannot determine as authorized for sale have been removed from our catalog in all Amazon markets and our warehouse. All ASINs were added in our Restricted Products List as a guarantee that they will not be listed on Amazon again. Removal orders have been created and completed:
• Removal orders ID: 240801YMO_0, 240801YMM_0, 240801KZW_0
C) Preventive measures implemented:
We have implemented a comprehensive verification process for our products by deleting all inventory on this marketplace to ensure there are no further policy violations before confirming compliance of all items and their active ingredients with Amazon policies and applicable laws.
Summary list of Prohibited Products or ASINs and Active Ingredients such as CBD (Cannabidiol) and similar has been created to prevent the inadvertent entry of such Restricted Products into the catalog.
We have created an Active Ingredients Database for each product in our catalog including a Safety Data Sheet (SDS). Also we have confirmation of compliance from at least 2 employees.
We have introduced a regular practice of extensive training for all employees regarding Category, Product and Content Restrictions and all Amazon’s policies. We now consult Amazon’s guidelines and external sources rigorously before listing any new products.
I have sought counsel from legal and medical experts to verify the legality of each product in our warehouse and products that cannot be confirmed as compliant with Amazon policies and applicable laws in the country will not be listed.
Build International Listing (BIL) connection was removed to prevent products from being inadvertently listed without proper oversight from at least 2 employees
The supplier of the prohibited products has been notified of the non-compliance. We have discontinued it as a precaution to ensure that we do not stock products from the restricted product list.
All new products will undergo thorough verification by myself and confirmed in compliance with Restricted Product Lists and Active Ingredients Lists before being listed.
Regular Training sessions on Amazon’s policies, particularly Restricted Products, are introduced every 14 days.
I am confident that these actions address the issue comprehensively and will prevent any future policy violations. I appreciate your consideration and look forward to your positive response.
This is the answer.
What we have to do in this case ? What we have to change in our PoA or we need to submit documents ?